
This equation is not balanced because there are an unequal amount of H's, N's and O's on both sides of the equation. However, the left hand side has 1 H, 1 N, and 3 O's and the right hand side has 2 H's, 3 N's and 9 O's. The left hand side has 1 Cu and the right hand side has 1 Cu, so the Cu atoms are balanced. Now that we've hopefully gotten the hang of balancing simple chemical equations, lets move onto something a bit more complex, such as the reaction of Copper (Cu) and Nitric Acid (HNO3) into Copper(II) Nitrate (Cu(NO3)2) + Nitrogen Dioxide (NO2) + Water (H2O):
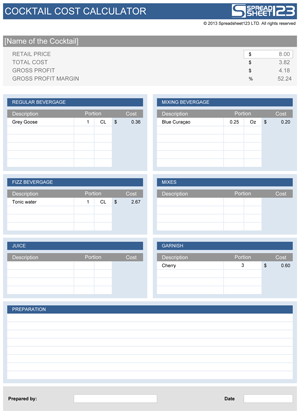
Cocktail chemistry calculator trial#
We could have tried, but we would have realized that we would need to balance H again (hence the trial and error). You might wonder why we didn't instead increase the number of H2 molecules. As a next step, lets balance H again by putting a 2 in front of NaOH so the equation reads:
Cocktail chemistry calculator update#
This is common and doesn't mean any mistakes were made.Īs was mentioned before, we knew we had to update multiple coefficients to balance the hydrogen atoms. We went from 1 unbalanced element to multiple. We now have 4 H, 2 O and 1 Na atom on the left, but 3 H, 1 O and 1 Na atom on the right. Notice how the 2 in front of H2O is "distributed" to both the H 2 and the O. Because 3 is not divisible by 2, it means coefficients of multiple compounds containing H will need to be changed.

In this case, there are an equal number of Na and O atoms, but like last time, H needs to be balanced, with 2 on the left, and 3 on the right. Take for example the exothermic reaction of Sodium (Na) and Water (H2O), which releases heat, Sodium Hydroxide (NaOH) and Hydrogen Gas (H2): Usually, balancing chemical equations will require multiple steps. The previous example was simpler than most but demonstrated the basic concepts.

That is the case in chemical equations like:

Balance Using Inspection + Linear Systems.


 0 kommentar(er)
0 kommentar(er)
